Pilot Study by Liquid Biopsy in Gastrointestinal Stromal Tumors: Analysis of PDGFRA D842V Mutation and Hypermethylation of SEPT9 Presence by Digital Droplet PCR
Abstract
:1. Introduction
2. Results
2.1. Patient and Sample Characteristics
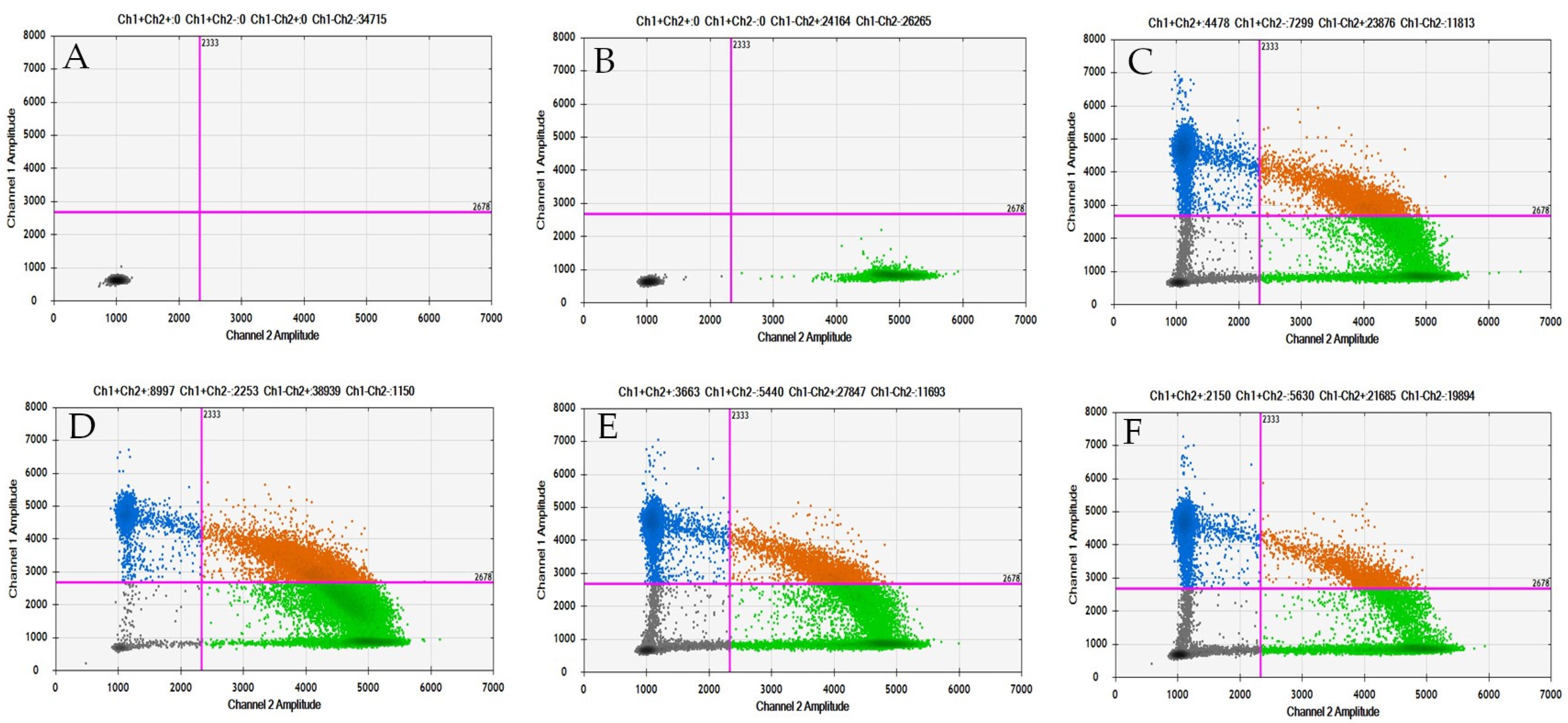
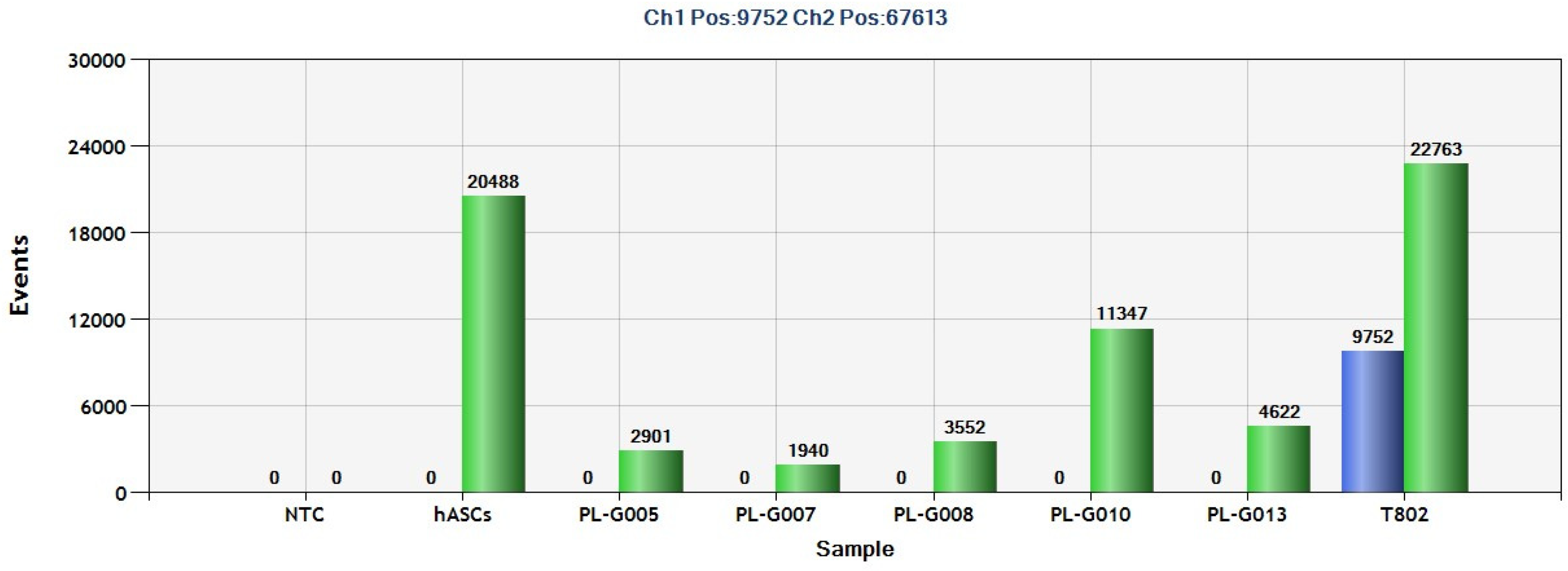
2.2. Detection of PDGFRA D842V Mutation in GIST Tissue Tumors Using ddPCR
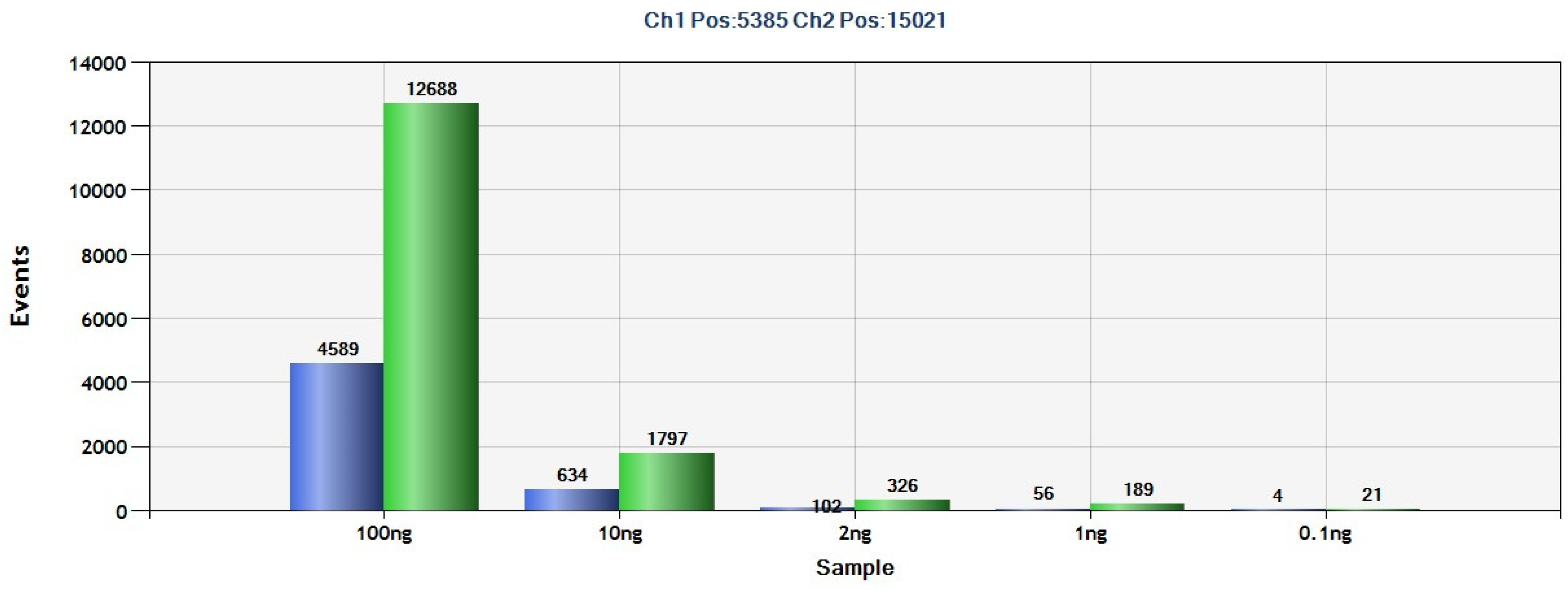
2.3. Detection of PDGFRA D842V Mutation in Plasma from GIST Patients by ddPCR
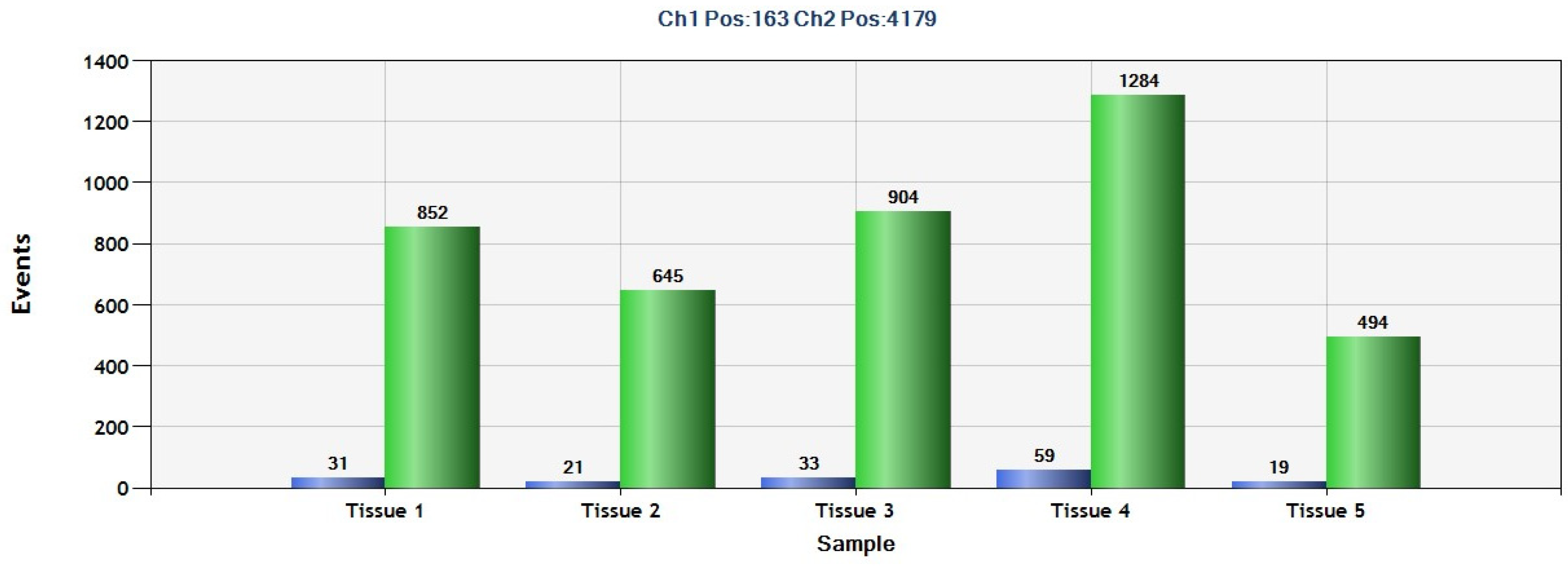
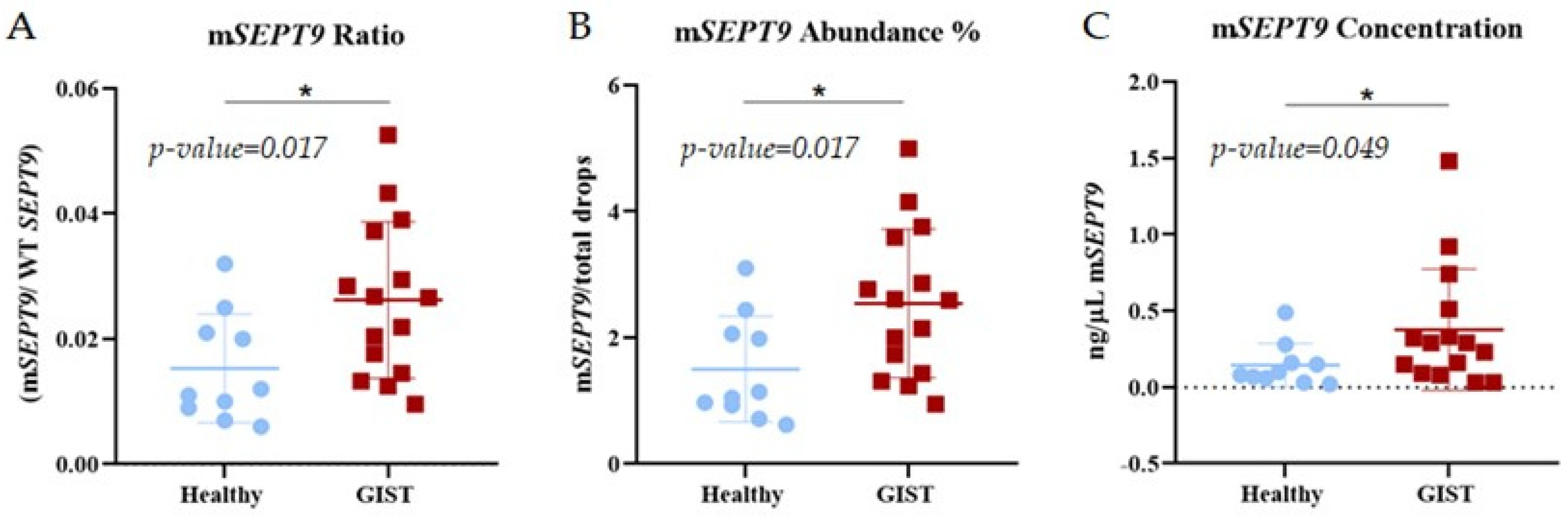
2.4. Detection of SEPT9 Hypermethylation in GIST Tissue by ddPCR
2.5. Detection of SEPT9 Hypermethylation in Plasma from GIST Patients by ddPCR
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. DNA Isolation
4.2.1. GIST Tissue
4.2.2. Plasma Samples
4.3. Bisulfite Treatment
4.4. Droplet Digital PCR (ddPCR)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Yin, Y.; Wang, X.; Yang, C.; Wan, S.; Yin, X.; Wu, T.; Chen, H.; Xu, Z.; Li, X.; et al. Gastrointestinal Stromal Tumors: Associations between Contrast-Enhanced CT Images and KIT Exon 11 Gene Mutation. Ann. Transl. Med. 2021, 9, 1496. [Google Scholar] [CrossRef]
- Akahoshi, K.; Oya, M.; Koga, T.; Shiratsuchi, Y. Current Clinical Management of Gastrointestinal Stromal Tumor. World J. Gastroenterol. 2018, 24, 2806–2817. [Google Scholar] [CrossRef]
- Boonstra, P.A.; Gietema, J.A.; Suurmeijer, A.J.H.; Groves, M.R.; de Assis Batista, F.; Schuuring, E.; Reyners, A.K.L. Tyrosine Kinase Inhibitor Sensitive PDGFRA Mutations in GIST: Two Cases and Review of the Literature. Oncotarget 2017, 8, 109836–109847. [Google Scholar] [CrossRef]
- Dalle Fratte, C.; Guardascione, M.; De Mattia, E.; Borsatti, E.; Boschetto, R.; Farruggio, A.; Canzonieri, V.; Romanato, L.; Borsatti, R.; Gagno, S.; et al. Clonal Selection of a Novel Deleterious TP53 Somatic Mutation Discovered in ctDNA of a KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumor Resistant to Imatinib. Front. Pharmacol. 2020, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.X.; Devine, C.; Segaran, N.; Ganeshan, D. Current Update on Molecular Cytogenetics, Diagnosis and Management of Gastrointestinal Stromal Tumors. World J. Gastroenterol. 2021, 27, 7125–7133. [Google Scholar] [CrossRef]
- George, S.; von Mehren, M.; Fletcher, J.A.; Sun, J.; Zhang, S.; Pritchard, J.R.; Hodgson, J.G.; Kerstein, D.; Rivera, V.M.; Haluska, F.G.; et al. Phase II Study of Ponatinib in Advanced Gastrointestinal Stromal Tumors: Efficacy, Safety, and Impact of Liquid Biopsy and Other Biomarkers. Clin. Cancer Res. 2022, 28, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Wasag, B.; Debiec-Rychter, M.; Pauwels, P.; Stul, M.; Vranckx, H.; Oosterom, A.V.; Hagemeijer, A.; Sciot, R. Differential Expression of KIT/PDGFRA Mutant Isoforms in Epithelioid and Mixed Variants of Gastrointestinal Stromal Tumors Depends Predominantly on the Tumor Site. Mod. Pathol. 2004, 17, 889–894. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Corless, C.L.; Demetri, G.D.; Blanke, C.D.; von Mehren, M.; Joensuu, H.; McGreevey, L.S.; Chen, C.-J.; Van den Abbeele, A.D.; Druker, B.J.; et al. Kinase Mutations and Imatinib Response in Patients with Metastatic Gastrointestinal Stromal Tumor. J. Clin. Oncol. 2003, 21, 4342–4349. [Google Scholar] [CrossRef]
- Lasota, J.; Miettinen, M. KIT and PDGFRA Mutations in Gastrointestinal Stromal Tumors (GISTs). Semin. Diagn. Pathol. 2006, 23, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Serrano, C.; Vivancos, A.; López-Pousa, A.; Matito, J.; Mancuso, F.M.; Valverde, C.; Quiroga, S.; Landolfi, S.; Castro, S.; Dopazo, C.; et al. Clinical Value of next Generation Sequencing of Plasma Cell-Free DNA in Gastrointestinal Stromal Tumors. BMC Cancer 2020, 20, 99. [Google Scholar] [CrossRef]
- Indio, V.; Ravegnini, G.; Astolfi, A.; Urbini, M.; Saponara, M.; De Leo, A.; Gruppioni, E.; Tarantino, G.; Angelini, S.; Pession, A.; et al. Gene Expression Profiling of PDGFRA Mutant GIST Reveals Immune Signatures as a Specific Fingerprint of D842V Exon 18 Mutation. Front. Immunol. 2020, 11, 851. [Google Scholar] [CrossRef]
- Gómez-Peregrina, D.; García-Valverde, A.; Pilco-Janeta, D.; Serrano, C. Liquid Biopsy in Gastrointestinal Stromal Tumors: Ready for Prime Time? Curr. Treat. Options Oncol. 2021, 22, 32. [Google Scholar] [CrossRef]
- Huang, W.; Yuan, W.; Ren, L.; Liang, H.; Du, X.; Sun, X.; Fang, Y.; Gao, X.; Fu, M.; Sun, Y.; et al. Clinicopathological and Therapeutic Analysis of PDGFRA Mutated Gastrointestinal Stromal Tumor. Pathol.—Res. Pract. 2022, 239, 154138. [Google Scholar] [CrossRef]
- Kelly, C.M.; Gutierrez Sainz, L.; Chi, P. The Management of Metastatic GIST: Current Standard and Investigational Therapeutics. J. Hematol. Oncol. 2021, 14, 2. [Google Scholar] [CrossRef]
- Ravegnini, G.; Sammarini, G.; Serrano, C.; Nannini, M.; Pantaleo, M.A.; Hrelia, P.; Angelini, S. Clinical Relevance of Circulating Molecules in Cancer: Focus on Gastrointestinal Stromal Tumors. Ther. Adv. Med. Oncol. 2019, 11, 1758835919831902. [Google Scholar] [CrossRef]
- Chevrier, S.; Brasselet, A.; Carnet, M.; Chevriaux, A.; Gibeaud, A.; Jourdain, M.; Mananet, H.; Truntzer, C.; Beltjens, F.; Charon-Barra, C.; et al. Custom Multi-Tumor next-Generation Sequencing Panel for Routine Molecular Diagnosis of Solid Tumors: Validation and Results from Three-Year Clinical Use. Int. J. Mol. Med. 2022, 49, 57. [Google Scholar] [CrossRef]
- Boonstra, P.A.; Ter Elst, A.; Tibbesma, M.; Bosman, L.J.; Mathijssen, R.; Atrafi, F.; van Coevorden, F.; Steeghs, N.; Farag, S.; Gelderblom, H.; et al. A Single Digital Droplet PCR Assay to Detect Multiple KIT Exon 11 Mutations in Tumor and Plasma from Patients with Gastrointestinal Stromal Tumors. Oncotarget 2018, 9, 13870–13883. [Google Scholar] [CrossRef]
- Siravegna, G.; Mussolin, B.; Venesio, T.; Marsoni, S.; Seoane, J.; Dive, C.; Papadopoulos, N.; Kopetz, S.; Corcoran, R.B.; Siu, L.L.; et al. How Liquid Biopsies Can Change Clinical Practice in Oncology. Ann. Oncol. 2019, 30, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Kirchweger, P.; Wundsam, H.V.; Rumpold, H. Circulating Tumor DNA for Diagnosis, Prognosis and Treatment of Gastrointestinal Malignancies. World J. Clin. Oncol. 2022, 13, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Escudero, L.; Martínez-Ricarte, F.; Seoane, J. ctDNA-Based Liquid Biopsy of Cerebrospinal Fluid in Brain Cancer. Cancers 2021, 13, 1989. [Google Scholar] [CrossRef]
- Olmedillas-López, S.; García-Arranz, M.; García-Olmo, D. Current and Emerging Applications of Droplet Digital PCR in Oncology. Mol. Diagn. Ther. 2017, 21, 493–510. [Google Scholar] [CrossRef]
- Olmedillas López, S.; García-Olmo, D.C.; García-Arranz, M.; Guadalajara, H.; Pastor, C.; García-Olmo, D. KRAS G12V Mutation Detection by Droplet Digital PCR in Circulating Cell-Free DNA of Colorectal Cancer Patients. Int. J. Mol. Sci. 2016, 17, 484. [Google Scholar] [CrossRef]
- Olmedillas-López, S.; Olivera-Salazar, R.; García-Arranz, M.; García-Olmo, D. Current and Emerging Applications of Droplet Digital PCR in Oncology: An Updated Review. Mol. Diagn. Ther. 2021, 26, 61–87. [Google Scholar] [CrossRef]
- Jilg, S.; Rassner, M.; Maier, J.; Waldeck, S.; Kehl, V.; Follo, M.; Philipp, U.; Sauter, A.; Specht, K.; Mitschke, J.; et al. Circulating cKIT and PDGFRA DNA Indicates Disease Activity in Gastrointestinal Stromal Tumor (GIST). Int. J. Cancer 2019, 145, 2292–2303. [Google Scholar] [CrossRef]
- Maier, J.; Lange, T.; Kerle, I.; Specht, K.; Bruegel, M.; Wickenhauser, C.; Jost, P.; Niederwieser, D.; Peschel, C.; Duyster, J.; et al. Detection of Mutant Free Circulating Tumor DNA in the Plasma of Patients with Gastrointestinal Stromal Tumor Harboring Activating Mutations of CKIT or PDGFRA. Clin. Cancer Res. 2013, 19, 4854–4867. [Google Scholar] [CrossRef]
- Scott, M.; Hyland, P.L.; McGregor, G.; Hillan, K.J.; Russell, S.E.H.; Hall, P.A. Multimodality Expression Profiling Shows SEPT9 to Be Overexpressed in a Wide Range of Human Tumours. Oncogene 2005, 24, 4688–4700. [Google Scholar] [CrossRef]
- Li, W.; Ma, X.; Wang, F.; Chen, S.; Guo, Q.; Sun, F.; Duan, Y. SNHG3 Affects Gastric Cancer Development by Regulating SEPT9 Methylation. J. Oncol. 2022, 2022, 3433406. [Google Scholar] [CrossRef]
- Verbakel, J.Y.; Steyerberg, E.W.; Uno, H.; De Cock, B.; Wynants, L.; Collins, G.S.; Van Calster, B. ROC Curves for Clinical Prediction Models Part 1. ROC Plots Showed No Added Value above the AUC When Evaluating the Performance of Clinical Prediction Models. J. Clin. Epidemiol. 2020, 126, 207–216. [Google Scholar] [CrossRef]
- Blay, J.-Y.; Kang, Y.-K.; Nishida, T.; von Mehren, M. Gastrointestinal Stromal Tumours. Nat. Rev. Dis. Primers 2021, 7, 22. [Google Scholar] [CrossRef]
- Grützmann, R.; Molnar, B.; Pilarsky, C.; Habermann, J.K.; Schlag, P.M.; Saeger, H.D.; Miehlke, S.; Stolz, T.; Model, F.; Roblick, U.J.; et al. Sensitive Detection of Colorectal Cancer in Peripheral Blood by Septin 9 DNA Methylation Assay. PLoS ONE 2008, 3, e3759. [Google Scholar] [CrossRef]
- Anghel, S.A.; Ioniță-Mîndrican, C.-B.; Luca, I.; Pop, A.L. Promising Epigenetic Biomarkers for the Early Detection of Colorectal Cancer: A Systematic Review. Cancers 2021, 13, 4965. [Google Scholar] [CrossRef]
- Al-Share, B.; Alloghbi, A.; Al Hallak, M.N.; Uddin, H.; Azmi, A.; Mohammad, R.M.; Kim, S.H.; Shields, A.F.; Philip, P.A. Gastrointestinal Stromal Tumor: A Review of Current and Emerging Therapies. Cancer Metastasis Rev. 2021, 40, 625–641. [Google Scholar] [CrossRef]
- Ford, S.J.; Gronchi, A. Indications for Surgery in Advanced/Metastatic GIST. Eur. J. Cancer 2016, 63, 154–167. [Google Scholar] [CrossRef]
- Richter, T.; Nestler-Parr, S.; Babela, R.; Khan, Z.M.; Tesoro, T.; Molsen, E.; Hughes, D.A.; International Society for Pharmacoeconomics and Outcomes Research Rare Disease Special Interest Group. Rare Disease Terminology and Definitions—A Systematic Global Review: Report of the ISPOR Rare Disease Special Interest Group. Value Health 2015, 18, 906–914. [Google Scholar] [CrossRef]
- Ullman, T.A.; Itzkowitz, S.H. Intestinal Inflammation and Cancer. Gastroenterology 2011, 140, 1807–1816. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Chan, C.S.Y.; Lau, K.S.; Ng, L.; Cheng, Y.Y.; Leung, W.K. Application of Droplet Digital Polymerase Chain Reaction of Plasma Methylated Septin 9 on Detection and Early Monitoring of Colorectal Cancer. Sci. Rep. 2021, 11, 23446. [Google Scholar] [CrossRef]
| Code | DNA Alterations | Diagnosis | Age | Sex | Tumor Size (cm) | Tumor Stage |
|---|---|---|---|---|---|---|
| G001 | N/A 1 | Schwannoma | 50 | Female | 5 × 4 × 3 | N/A |
| G002 | KIT exon 11 (W557R y V555E) | Gastric GIST | 78 | Male | 3 × 3 × 2.5 | pT2N0 |
| G003 | KIT exon 11 (W557R) | Gastric GIST | 66 | Female | 4 × 4 × 3 | pT2N0 |
| G004 | N/A | Fibromyxoma | 59 | Female | 3 × 2 × 2 | N/A 1 |
| G005 | PDGFRA exon 18 (D842V) | Gastric GIST | 70 | Male | 2 × 2 × 2 | pT2N0 |
| G006 | PDGFRA exon 18 (D842_S847 del) | Gastric GIST | 78 | Female | 3 × 2 × 1 | pT1N0 |
| G007 | PDGFRA exon 18 (D842V) | Gastric GIST | 66 | Male | 3 × 2 × 2 | pT2N0 |
| G008 | PDGFRA exon 18 (D842V) | Gastric GIST | 71 | Male | 9 × 5 × 5 | N/A |
| G009 | KIT exon 11 (D579del) | Gastric GIST | 70 | Female | 5 × 4 × 4 | pT2N0 |
| G010 | No alterations in KIT and PDGFRA, WT | Gastric GIST | 86 | Female | 4 × 3 × 3 | pT2N0 |
| G011 | N/A | Lipoma | 58 | Female | 5 × 4 × 3 | N/A |
| G012 | KIT exon 11 (W557_K558del) | Gastric GIST | 54 | Male | 16 × 10 × 11 | pT4N0 |
| G013 | PDGFRA exon 18 (D842V) | Gastric GIST | 56 | Male | 4 × 3 × 3 | pT2N0 |
| G014 | KIT exon 11 (V559_E561del) | Gastric GIST | 52 | Female | 9 × 8 × 8 | pT3N0 |
| G015 | KIT exon 11 (W557G) | Gastric GIST | 68 | Male | 4 × 3 × 3 | pT2N0 |
| G016 | KIT exon 11 (G556_K558delinsE) | Gastric GIST | 62 | Female | 6 × 5 × 4 | YpT3N0 |
| G017 | KIT exon 11 (M552_K558) | Gastric GIST | 73 | Female | 4 × 3 × 2.5 | ypT2yPN0 |
| G018 | PDGFRA exon 18 (D842V) | Gastric GIST | 57 | Male | 5 × 5 × 1.5 | pT2PN0 |
| Tissue Coded DNA | Alteration PDGFRA | % Tumoral | ng/µL DNA | 260/280 QC DNA |
|---|---|---|---|---|
| AP 22155-802 | PDGFRA exon 18 (D842V) | 80 | 130 | 1.87 |
| AP 22155-803 | PDGFRA exon 18 (D842V) | 60 | 138.2 | 1.9 |
| AP 22155-804 | PDGFRA exon 18 (D842V) | 75 | 146 | 1.86 |
| AP 22155-805 | WT | 85 | 176.2 | 1.9 |
| AP 22155-806 | PDGFRA exon 18 (D842V) | 70 | 127.3 | 1.85 |
| Sample | ng/µL | WT PDGFRA Events Detected |
|---|---|---|
| Fresh plasma 3.5 mL | 3.52 | 351 |
| Cryopreserved plasma 3.5 mL | 1.35 | 108.83 |
| Large volume of cryopreserved plasma 12 mL | 4.57 | 340 |
| DNA concentrated by centrifugation | 1.87 | 48.75 |
| DNA concentrated by SpeedVacum | 1.46 | 48.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivera-Salazar, R.; Salcedo Cabañas, G.; Vega-Clemente, L.; Alonso-Martín, D.; Castellano Megías, V.M.; Volward, P.; García-Olmo, D.; García-Arranz, M. Pilot Study by Liquid Biopsy in Gastrointestinal Stromal Tumors: Analysis of PDGFRA D842V Mutation and Hypermethylation of SEPT9 Presence by Digital Droplet PCR. Int. J. Mol. Sci. 2024, 25, 6783. https://doi.org/10.3390/ijms25126783
Olivera-Salazar R, Salcedo Cabañas G, Vega-Clemente L, Alonso-Martín D, Castellano Megías VM, Volward P, García-Olmo D, García-Arranz M. Pilot Study by Liquid Biopsy in Gastrointestinal Stromal Tumors: Analysis of PDGFRA D842V Mutation and Hypermethylation of SEPT9 Presence by Digital Droplet PCR. International Journal of Molecular Sciences. 2024; 25(12):6783. https://doi.org/10.3390/ijms25126783
Chicago/Turabian StyleOlivera-Salazar, Rocío, Gabriel Salcedo Cabañas, Luz Vega-Clemente, David Alonso-Martín, Víctor Manuel Castellano Megías, Peter Volward, Damián García-Olmo, and Mariano García-Arranz. 2024. "Pilot Study by Liquid Biopsy in Gastrointestinal Stromal Tumors: Analysis of PDGFRA D842V Mutation and Hypermethylation of SEPT9 Presence by Digital Droplet PCR" International Journal of Molecular Sciences 25, no. 12: 6783. https://doi.org/10.3390/ijms25126783
APA StyleOlivera-Salazar, R., Salcedo Cabañas, G., Vega-Clemente, L., Alonso-Martín, D., Castellano Megías, V. M., Volward, P., García-Olmo, D., & García-Arranz, M. (2024). Pilot Study by Liquid Biopsy in Gastrointestinal Stromal Tumors: Analysis of PDGFRA D842V Mutation and Hypermethylation of SEPT9 Presence by Digital Droplet PCR. International Journal of Molecular Sciences, 25(12), 6783. https://doi.org/10.3390/ijms25126783







